Here is an in-depth look at silver the element:
-
Basic Information:
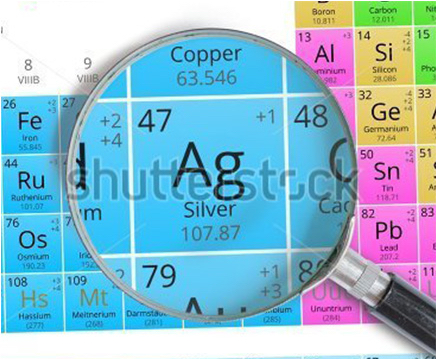
- Symbol: Ag
- Atomic number: 47
- Group number: 11
- Mass: 107.868
- Density @ 293 K: 10.5 g/cm3
- Atomic volume: 10.3 cm3/mol
- Melting Point: 961.93 C (1235.1 K)
- Boiling Point: 2212 C (2428 K)
- Heat of fusion: 11.30 kJ/mol
- Heat of vaporization: 250.580 kJ/mol
- Number of Protons/Electrons: 47
- Number of neutrons: 61
- Classification: Transition Metal
- Crystal Structure: Face-centered Cubic
- Color: silver
- Hardness: 3.25 mohs
- Characteristics: soft, ductile, tarnishes
-
Structure of atom:
-

-
Oxidation:
- Electron configuration: [Kr] 4d10 5s1
- Minimum oxidation number: 0
- Maximum oxidation number: 3
- Minimum oxidation state: 0 (silver occurs naturally in ores in its elemental state)
- Maximum oxidation state: 3 (the unit cell of silver oxide, Ag4O4, has two atoms of univalent silver and two atoms of trivalent silver)
-
Half Lives:
-
Energies:

Recent Comments